|
Introduction: The Lesotho Ministry of Health and Social Welfare’s (MOHSW) 5-year strategic plan, as well as their national laboratory policy and yearly operational plans, directly addresses issues of
accreditation, indicating their commitment to fulfilling their mandate. As such, the MOHSW adopted the World Health Organization Regional Headquarters for Africa's Stepwise Laboratory Quality Improvement
Toward Accreditation (WHO–AFRO–SLIPTA) process and subsequently rolled out the Strengthening Laboratory Management Towards Accreditation (SLMTA) programme across the whole country, becoming the first
African country to do so.Methods: SLMTA in Lesotho was implemented in two cohorts. Twelve and nineteen laboratory supervisors and quality officers were enrolled in Cohort 1 and Cohort 2, respectively. These 31 participants
represented 18 of the 19 laboratories nationwide. For the purposes of this programme, the Queen Elizabeth II (QE II) Central Laboratory had its seven sections of haematology, blood bank, cytology, blood
transfusion, microbiology, tuberculosis laboratory and chemistry assessed as separate sections. Performance was tracked using the WHO–AFRO-SLIPTA checklist, with assessments carried out at baseline and
at the end of SLMTA. Two methods were used to implement SLMTA: the traditional ‘three workshops’ approach and twinning SLMTA with mentorship. The latter, with intensive follow-up visits, was
concluded in 9 months and the former in 11 months. A standard data collection tool was used for site visits. Results: Of the 31 participants across both cohorts, 25 (81%) graduated (9 from Cohort 1 and 16 from Cohort 2). At baseline, all but one laboratory attained a rating of zero stars, with the exception
attaining one star. At the final assessment, 7 of the 25 laboratories examined at baseline were still at a rating of zero stars, whilst 8 attained one star, 5 attained two stars and 4 attained three stars.
None scored above three stars. The highest percentage improvement for any laboratory was 51%, whereas the least improved dropped by 6% when compared to its baseline assessment. The most improved areas were
corrective actions (34%) and documents and records (32%). Process improvement demonstrated the least improvement (10%). Conclusion: The SLMTA programme had an immediate, measurable and positive impact on laboratories in Lesotho. This success was possible because of the leadership and ownership of the programme by the
MOHSW, as well as the coordination of partner support.
The Lesotho Ministry of Health and Social Welfare (MOHSW), through its Laboratory Services
Directorate, is committed to the provision of essential services as part of the national
health-care delivery to all Basotho people. These services include comprehensive diagnostic
testing of all prevalent major infections, for example, HIV and TB, monitoring of patient
treatment, drug resistance testing and surveillance studies that inform policymaking decisions
and major health reform.1The laboratory system in Lesotho is structured in three tiers: referral, regional and district
laboratories. The Central Laboratory at the Queen Elizabeth II (QE II) Hospital in Maseru serves
as the national reference laboratory. There are two regional laboratories. The other 16
laboratories are at the district level, with 7 of these managed by the MOHSW, 1 by the military
and 7 by the Christian Health Association of Lesotho. The final district laboratory is owned by
the Partners in Health, anon-governmental organisation. There has been a significant and progressive increase in demand for laboratory services in Lesotho,
with the QE II Central Laboratory testing 114 114 specimens in 2006, compared to 16 250 in 2003,
a 600% increase.2 The MOHSW realised that the increased
demand for testing had to be matched with high quality testing. Accreditation was identified
as one means of assuring continuous quality testing services.3 The MOHSW and its laboratory partners have put in place a number of key pillars for launching
their bid to accredit the public health and clinical laboratories successfully. These include
a national laboratory policy, a 5-year national strategic plan, the appointment of a laboratory
director and a national Quality Assurance Unit (QAU) headed by a national quality manager who
is supported by key national quality officers. The QAU is a unit created by
Laboratory Services to manage the quality improvement initiatives for the entire laboratory network.
In the strategic plan, Objective 2.3 directly addresses these initiatives, aiming ‘to
strengthen quality assurance of Laboratory Services and have a mechanism for attaining international
accreditation defined’.3 These clearly stated objectives have been translated into yearly
operational plans for the last 3 years,2 culminating, in 2011, in the generation of
a ground breaking yearly operational plan that aimed to have seven laboratories declared ready
for the World Health Organization Regional Headquarters for Africa Stepwise Laboratory Quality Improvement Toward Accreditation (WHO–AFRO–SLIPTA) process by the 3rd Quarter
of that year.2 This target was achieved but, unfortunately, the WHO–AFRO–SLIPTA
office was not ready to accept applications by that time. The QAU was therefore established to ensure
continued support for these quality improvement efforts. The Strengthening Laboratory Management Towards Accreditation (SLMTA) programme
was launched concurrently with the stepwise WHO–AFRO–SLIPTA process
in Kigali, Rwanda in 2009.4 SLMTA, a
task-based curriculum, assists countries in the training
of laboratory managers to implement the quality management system requirements of the WHO–AFRO–SLIPTA process,
with the aim of granting them eventual international accreditation.5 The MOHSW of Lesotho immediately embraced the SLMTA programme soon after engaging in
the trainers’ workshopheld at the African Centre of Integrated Laboratory
Training in Johannesburg, South Africa in November 2009. This programme was implemented
at an opportune time in Lesotho, because the country had already embarked on laboratory
improvements through a number of policies and critical documents such as the national
laboratory policy and 5-year strategic plan. A number of critical officers, namely the
laboratory director, quality manager, national safety officer, national training officer,
as well as programmes such as mentorship, were also in place. The SLMTA programme in
Lesotho was coordinated by the MOHSW with the SLMTA coordinator facilitating as one of the quality officers within the QAU.
A number of laboratory partners, namely the Association of Public Health Laboratories (APHL), the Centers for Disease
Control and Prevention (CDC) and the Clinton Health Access Initiative (CHAI), have provided technical and logistical
support to the programme. The SLMTA programme has been part of the Laboratory Services’ yearly operational
plans for the past 2 years.2 This paper describes the experience of Lesotho in implementing the SLMTA programme.
The purpose is to share this experience and the lessons learned from it with other
countries that are implementing, or planning to implement, the SLMTA programme.
The analysis of the programme in this paper will also inform present programme
activities in Lesotho, as plans for the training of more cohorts across the country are already underway.
The SLMTA programme in Lesotho was implemented in two cohorts, with Cohort 1 comprising 12 participants.
These 12 participants selected from 4 district laboratories, 7 QE II Central Laboratory sections
(chemistry, haematology, cytology, microbiology, blood bank, blood transfusion and the TB laboratory) and
1 quality officer from the QAU. All four participants from district laboratories were laboratory managers,
whilst three of the seven from the QE II Central Laboratory were section supervisors and the other four were
section level quality officers (Table 1). Cohort 1 enrolled only laboratories that were located within the
vicinity of Maseru, the location of the training venue.
|
TABLE 1: Profile of Cohort 1 and Cohort 2 participants in the Strengthening Laboratory
Management Towards Accreditation (SLMTA) programme in Lesotho.
|
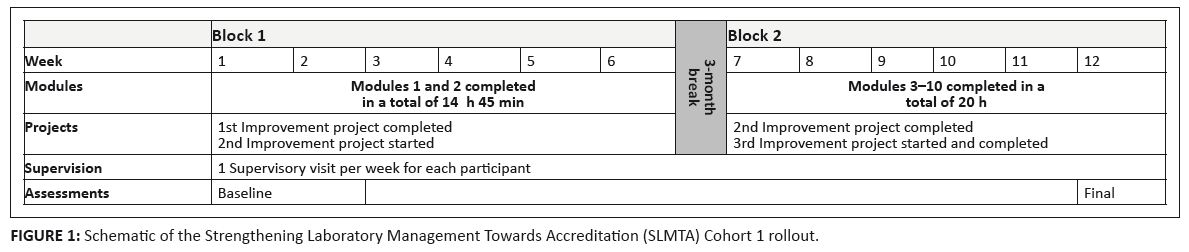 |
FIGURE 1: Schematic of the Strengthening Laboratory Management Towards Accreditation (SLMTA) Cohort 1 rollout.
|
|
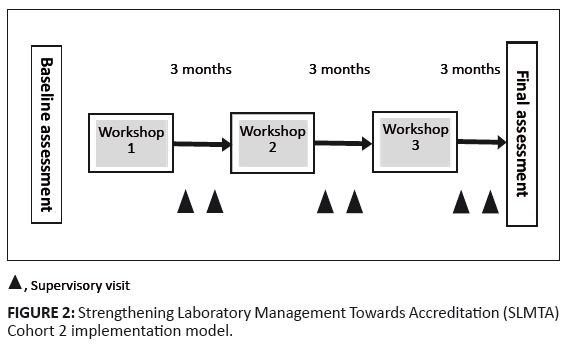 |
FIGURE 2: Strengthening Laboratory Management Towards Accreditation (SLMTA) Cohort 2 implementation model.
|
|
Cohort 1 did not follow the three workshop series recommended for SLMTA; instead, SLMTA was twinned with mentorship,
which was already under way. Seven of the participants in this cohort came from the QE II Central Laboratory, where
the mentor, who was also the SLMTA facilitator, was conducting the second round of mentorship at that time.
The other four were based within an 80 km radius of the Central Laboratory, whilst the participant from the
QAU was based within the MOHSW headquarters, the training venue.Instead of the recommended 4–5 day workshops, the SLMTA modules were delivered 1 day per week
on a Friday over two blocks of 6 weeks each. The two blocks were spaced 3 months apart. In total,
the 1-day workshops over 12 weeks matched the 12 days of the recommended 4-day workshops, after
the three-workshop series was completed (Figure 1). More intensive follow-ups were feasible because of the
proximity of all participants. Each of the participants had one follow-up visit a week, to a total of 12
visits each over a 9-month period. The intensity of the follow-up visits allowed the participants to
complete the recommended three improvement projects over the course of 9 months. The increased
supervisory visits also allowed most of the participants to have more than one project running at a time;
for example, if one was waiting for supply requisition documents from procurement, another project could be initiated on sample rejections. Cohort 2 followed the recommended SLMTA three workshops approach (Figure 2), with three certified SLMTA facilitators affiliated with the MOHSW, APHL and CHAI. A total of 16 district
laboratories and 3 QE II Central Laboratory sections (each with 1 participant per laboratory, or per section, enrolled) were part of the SLMTA Cohort 2 (Table 1). Only one laboratory,
Quithing District Laboratory, did not attend the training because the communication for workshop attendance did not arrive on time. An implementation plan was developed before the programme started to ensure that the SLMTA programme for Cohort 2 had specific, fixed dates of activities spanning the entire 12 months. These were adhered to 98% of the time; that is, only one of the planned activities did not take place on the assigned date. The last activity not met was the assessment by WHO for the SLIPTA star status recognition, as the structures for such assessments were not in place by March 2010 (Table 2).
Baseline assessments
Baseline assessments were conducted by the three SLMTA facilitators using the WHO–AFRO–SLIPTA checklist.
Two facilitators assessed six laboratories each, whilst one assessed seven. Training on the use of the checklist was
conducted for two of the facilitators by the other facilitator who was a WHO trained assessor.
The WHO–AFRO–SLIPTA checklist provides a quantitative measure of adherence to
accreditation requirements for quality and competency. The scored checklist (totalling 250)
allows for the rating of a laboratory’s quality improvement status by using a zero–five star rating,
calculated as follows: 0–137 = zero stars, 138–160 = one star,
161–185 = two stars, 186–211 = three stars,
212–236 = four stars, and 237–250 = five stars.
Improvement projects
Baseline assessments were conducted by the three SLMTA facilitators using the WHO–AFRO–SLIPTA checklist.
Two facilitators assessed six laboratories each, whilst one assessed seven. Training on the use of the checklist
was conducted for two of the facilitators by the other facilitator who was a WHO trained assessor. The WHO–AFRO–SLIPTA
checklist provides a quantitative measure of adherence to accreditation requirements for quality and competency. The scored checklist
(totalling 250) allows for the rating of a laboratory’s quality improvement status by using a zero–five star rating,
calculated as follows: 0–137 = zero stars, 138–160 = one star, 161–185 = two stars,
186–211 = three stars, 212–236 = four stars, and 237–250 = five stars.
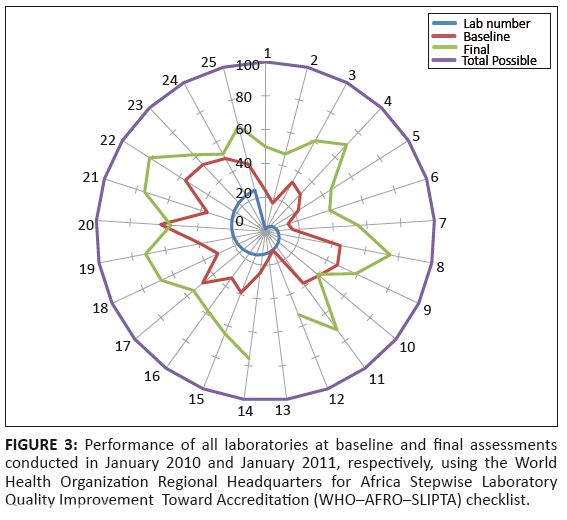 |
FIGURE 3: Performance of all laboratories at baseline and final assessments
conducted in January 2010 and January 2011, respectively, using the World
Health Organization Regional Headquarters for Africa Stepwise Laboratory
Quality Improvement Toward Accreditation (WHO¢AFRO¢SLIPTA) checklist.
|
|
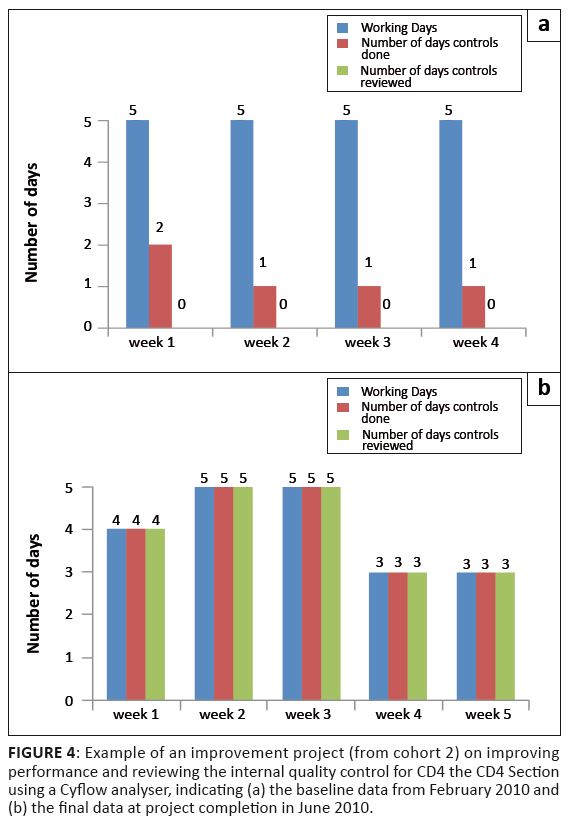 |
FIGURE 4: Example of an improvement project (from cohort 2) on improving
performance and reviewing the internal quality control for CD4 the CD4 Section
using a Cyflow analyser, indicating (a) the baseline data from February 2010 and
(b) the final data at project completion in June 2010.
|
|
Follow-up visits
For Cohort 2, each of the three facilitators was assigned laboratories which they
followed throughout the duration of the programme. Each facilitator followed up
with both the laboratories and the QE II Central Laboratory sections they had assessed at baseline.
This allowed for relationship building between the facilitator and the laboratory. During each visit,
the facilitator made an appointment with the hospital management to explain the vision of the
Laboratory Directorate of Accreditation, as well as the SLMTA process. The need to support the
laboratory was also emphasised.A SLMTA follow-up visit assessment tool was developed and implemented for
Cohort 2 to standardise follow-up visits and guide the facilitator on areas
that needed to be covered during the site visit. These included the provision
of supervision on the improvement project, follow-up on the implementation of
activities taught during the last workshop by determining the uptake of SLMTA tools,
the tracking of quality indicators and the implementation of a set of agreed compulsory activities.
Compulsory activities were considered to be the ‘must do’ and easy-to-implement activities
that did not constitute an improvement project, for example, a duty roster, an equipment master list,
a team meeting and the use of a management calendar. In addition, the tool required the facilitator
to document coaching provided to the SLMTA participant or other staff with regard to the improvement project,
as well as in relation to other areas of laboratory improvement. Each visit lasted one full working day.
All reports from visits were submitted to the SLMTA coordinator at the MOHSW, who ensured that the
participating laboratories received copies of their progress reports.
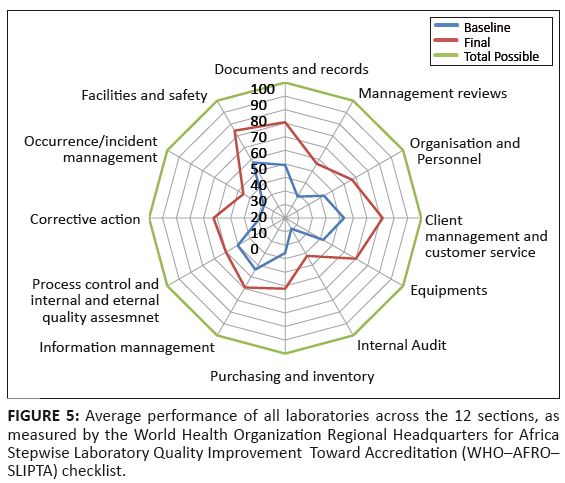 |
FIGURE 5: Average performance of all laboratories across the 12 sections, as
measured by the World Health Organization Regional Headquarters for Africa
Stepwise Laboratory Quality Improvement Toward Accreditation (WHO¢AFRO¢
SLIPTA) checklist.
|
|
|
TABLE 2: Lesotho Strengthening Laboratory Management Towards Accreditation
(SLMTA) Cohort 2 implementation plan, developed before the start of the
programme.
|
|
TABLE 3: List of improvement projects carried out by Cohort 1 and Cohort 2 participants in the Strengthening Laboratory Management Towards Accreditation (SLMTA)
programme in Lesotho.
|
A total of 12 participants were enrolled for Cohort 1 (Table 1), of whom 3 (25%) did not graduate.
Of the nine who graduated, four were from each of the four district laboratories,
four where affiliated with the QE II Central Laboratory sections of cytology,
haematology, microbiology and blood transfusion, and one was from the QAU (Table 1).
Of the three who did not graduate, one was transferred during the course of the
training and could not continue, whilst the other two did not meet the requirements
of 100% attendance and the completion of three improvement projects.For Cohort 2, 19 participants were enrolled (Table 1), of whom 16 (84%) graduated.
Three did not graduate because of reasons ranging from non-completion of
three improvement projects and attending less than three workshops, to one participant being placed under disciplinary suspension. A total of 25 participants successfully completed the SLMTA programmes for Cohorts 1 and 2.
These 25 were from 18 of the 19 laboratories of Lesotho. As mentioned above, the nineteenth
laboratory, Quithin, missed the first workshop as a consequence of miscommunication of training dates.
For assessment purposes, the QE II Central Laboratory had its seven sections of haematology,
blood bank, cytology, blood transfusion, microbiology, TB laboratory and chemistry classified
as separate sections; hence, the 25 assessments results in Figure 3.
Improvement project outcomes
For Cohort 1, the participant from the QAU received the award for the best improvement projects.
The selection was based on the overall impact that the participant’s three projects had on
the entire laboratory service of Lesotho. The winner’s first project resulted in the upgrade
and implementation of the current document control system for the entire network. Their second project
improved the tracking of equipment down-time and the reporting of equipment breakdown for all contracted
equipment within the government laboratory network, whilst their third project designed a mechanism of
providing assessment for laboratories that miss the external quality assurance deadlines for various reasons.In Cohort 2, the Butha Buthe District Laboratory received the award for the best improvement project,
which investigated the improvement of documentation in internal quality control for its CD4 section.
The difference between baseline and final data collected between February and June 2010 for the Butha
Buthe improvement project is illustrated in Figure 4. The overall performance of laboratories over the entire SLMTA period improved over time (Figure 3). Of the
25 laboratories, 24 (96%) demonstrated an improvement over the 12-month period. One section at Queen II Central
(Laboratory 13, Figure 3) was not assessed post-SLMTA because of miscommunication with the laboratory manager,
which resulted in the assessor being unable to access the laboratory. The most improved laboratory demonstrated
an increase of 51% from its baseline to final assessments, whilst the laboratory that performed the least demonstrated
a 6% drop between these two assessments. The average performance of the 25 laboratories across the 12 sections of
the WHO–AFRO–SLIPTA checklist is illustrated in Figure 5.
The most improved areas – measured by the difference between baseline
and final percentage score for each of the 12 sections – were corrective
actions (34%), documents and records (32%), and customer service (29%). The process
improvement category demonstrated the least improvement (10%). The star rating of the
laboratories using the WHO–AFRO–SLIPTA checklist star rating is reflected
in Table 3. At baseline, all but one laboratory had zero stars; yet, by the end of SLMTA,
17 (68%) of the laboratories had achieved a star status. Of that 17, 8 (32%) had one star, 5 (20%)
had two stars, and 4 (16%) had three stars, gained over a 12-month period of SLMTA (Table 4).
|
TABLE 4: Star rating of all laboratories between baseline and final assessments
using the World Health Organization Regional Headquarters for Africa Stepwise
Laboratory Quality Improvement Toward Accreditation (WHO¢AFRO¢SLIPTA)
checklist.
|
Tracking of performance data by using the WHO–AFRO–SLIPTA checklist showed that only one (14%)
of the 25 enrolled laboratories had at least a star one status rating at baseline out of a possible 5 stars after this word. However, by January 2011,
17 (68%) had achieved a star rating, with four of the laboratories reaching three-star status. The results
indicate that there was a measurable improvement over the 12-month period of SLMTA.One laboratory (Laboratory 20, Figure 3) had a negative improvement of 6% because of an unexpected
staff departure: the supervisor, who was in the SLMTA programme, was transferred to another duty station.
Then, of the two technologists from Laboratory 20 who remained, one went on extended maternity leave. Even
though systems could have been implemented, sustainment would have been difficult with only one out of
three possible staff members in place. In addition, the Tebellong District Laboratory (Laboratory 10, Figure 3)
had the smallest percentage of positive improvement, namely 1%, because of the unexpected withdrawal of an SLMTA participant.
The participant had to be withdrawn from SLMTA because of his disciplinary suspension from the hospital and, as such, he could
not attend Workshops 2 and 3. Tebellong had a staff complement of only two technologists. Replacement processes are carried out
centrally at the MOHSW public services department and the process takes at least 6 months. Laboratories, in general, were weaker in some areas than others. In particular, internal audits,
management reviews, corrective actions and process improvement, showed the lowest average scores.
These areas have been strengthened in SLMTA Cohort 3, currently in progress, as well as in the on-going mentorship programme. One of the strongest pillars of success of SLMTA in Lesotho was the strong commitment shown
by the Ministry of Health Laboratory Services to the SLMTA programme. The ownership and the
strong leadership of the Directorate of Laboratory Services and its coordination of technical
support by laboratory partners made the SLMTA successful in Lesotho. The high level of dedication
demonstrated by SLMTA participants created tremendous enthusiasm within the laboratories,
as observed by the three facilitators during the supervisory visits and workshop training.
This also might have contributed to improvements, despite ever-increasing workloads. Planning the entire SLMTA programme from the start helped to ensure that the programme was
completed on time with few logistical problems. The entire 12-month programme for Cohort 2
was designed in January 2010, with fixed dates of baseline assessments, all three workshops,
six follow-up visits and the final assessments decided upon at that time. This was critical,
because SLMTA is a long and continuous process and therefore chances of disruptions are high.
In this phase of SLMTA, 98% of the planned activities were met within the agreed timetable. Coordination by the MOHSW was central to the success of SLMTA and functioned as a means
of strengthening local capacity building within the QAU. Findings from the SLMTA assessments
and site visits informed the QAU on priority areas. Standardising the supervisory visits and
the meetings of hospital management for support was also critical for the improvement efforts.
The MOHSW and its partners involved in the SLMTA as facilitators had to have dedicated time
for the programme. The technical support and effective coordination of activities with the
MOHSW by its partners (CHAI, APHL and CDC) also played a pivotal role in the rollout of the SLMTA programme.
The SLMTA programme in Lesotho resulted in immediate, measurable laboratory
improvements shown by all but one laboratory. With this performance, seven
have been prepared and are ready for application to the WHO–AFRO–SLIPTA process.4 The
seven selected are those with the highest WHO–AFRO–SLIPTA checklist marks from the SLMTA final assessments conducted
by facilitators. As part of the preparation, these seven laboratories have already been assessed by WHO–AFRO–SLIPTA
trained assessors who were invited to Lesotho in February 2011.The Lesotho experience demonstrated that if SLMTA is planned and executed appropriately with the minimum of
six follow-up visits and the three improvement projects, it will become an effective programme. Thus, it is
clear that the improvement projects and follow-up visits are the two critical pillars of the SLMTA programme. However, the programme did face some challenges. From the perception of the participants to the process,
it was indicated that improvement projects consumed a lot of time and could not be carried out during the
course of their normal working day. In some instances, participants had to request to be relieved from their
routine work to work on the improvement projects. Only a few of the laboratories had computers for typing
their projects and for drafting quality documents. Participants also felt that the time of 3 months
allocated for improvement projects was too short. Furthermore, as a result of the strict criteria for
SLMTA participation to graduation, there is a risk that participants may not be able to fulfil all criteria.
This could result in the exclusion of laboratories from the SLMTA programme and a missed opportunity for
laboratory improvement. This is another good reason to continue to roll out the SLMTA programme,
as this will allow for continuous and sustainable laboratory quality improvement. This is the
approach that Lesotho has taken by continuing the SLMTA training of more cohorts comprising
different participants from the same laboratories.
We would like to thank all laboratory supervisors who actively participated in the: SLMTA programme
and management who supported the implementation of SLMTA. The financial and technical support provided by the CDC, APHL and CHAI, is acknowledged.
Competing interests
The authors declare that they have no financial
or personal relationship(s) which may have inappropriately influenced them in writing this article.
Authors’ contributions
D.M. (Ministry of Health and Social Welfare) was the project leader as Director of Laboratory Services, Lesotho. T.M. (Clinton Health Access Initiative) was an SLMTA facilitator
for both Cohort 1 and 2 and wrote the manuscript. M.L. (Ministry of Health and Social Welfare) and K.L. (Association of Public Health Laboratories) were SLMTA facilitators for
Cohort 1. JW (Clinton Health Access Initiative), as the country director of CHAI, and Y.M. (Centers for Disease Control and Prevention),
as the CDC Laboratory Advisor, were the project coordinators of this project. M.L., K.L. and Y.M. reviewed the manuscript.
1. Lesotho Ministry of Health and Social Welfare. Laboratory services national policy, October 2008. Maseru. Unpublished.2. Lesotho Ministry of Health and Social Welfare. Laboratory services national yearly operational plan, 2009/2010 to 2010/2011.Maseru. Unpublished. 3. Lesotho Ministry of Health and Social Welfare. Laboratory services national strategic plan, 2008/2009 to 2012/2013. Maseru. Unpublished. 4. World Health Organization. [WHO representative’s office for Rwanda] [press release]. Kigali: WHO; 2009 Jul 27. French. 5. Yao K, McKinney B, Murphy A, et al. Improving quality management systems of laboratories in developing countries.
An innovative training approach to accelerate laboratory accreditation. Am J Clin Pathol. 2010;134(3):401–409.
http://dx.doi.org/10.1309/AJCPNBBL53FWUIQJ, PMid:20716796
|
